Support Payment Term:
HSBC Hong Kong
paypal
Alibaba Pay
Western Union
USD
EUR
GBP
SGD
HKD
CNH
CAD
MXN
BRL
JPY
THB
MOP
AUD
NZD
PLN
CZK
HUF
RON
CHF
SEK
NOK
DKK
TRY
AED
SAR
ILS
ZAR
$0.00
Discover similar items
Page 1 of 2
Feline Herpesvirus Antigen Rapid Test Kit (FHV Ag) (Latex Immunochromatography)
Product Code:
E-FHV-01
Packaging Specification:
20 tests/box
Intended Use:
This product is used for qualitative detection of feline herpesvirus antigen in canine ocular and conjunctival secretions, nasal discharge, saliva, and feces samples. It is intended for initial screening of feline herpesvirus infections.
Feline herpesvirus, also known as viral rhinotracheitis, is a highly contagious pathogen that replicates and proliferates in the conjunctival and upper respiratory epithelial cells of cats worldwide. The virus enters the cat's body through the nose, mouth, and conjunctiva, causing lytic infections in nasal mucosal epithelial cells and spreading to the conjunctiva, throat, trachea, bronchi, and bronchioles.
Test Principle:
This reagent employs a double antibody sandwich method to detect feline herpesvirus antigen. It contains antibodies against feline herpesvirus antigen pre-fixed on the membrane test zone (T), latex-labeled antibodies against feline herpesvirus antigen, and secondary antibodies pre-fixed on the membrane control zone (C). When an appropriate amount of sample is added to the sample well of the test card, the sample moves forward along the test card. If the sample contains feline herpesvirus antigen, the latex-labeled antibodies will bind to it, forming an antigen-antibody-latex complex. During chromatography, this complex binds to the feline herpesvirus antibodies fixed on the membrane, resulting in a red band appearing in the test zone (T). If the sample does not contain feline herpesvirus antigen, no red band will appear in the test zone. Regardless of the presence of viral antigen in the sample, a red band will always appear in the control zone (C). If the control line does not appear, the test result is invalid, and the sample should be retested.
Main Components:
- Feline Herpesvirus Antigen (FHV Ag) Test Card
- Sample Diluent
- Disposable Pipette
- Sample Collection Swab
- User Manual (1 copy)
Storage Conditions and Shelf Life:
The test card should be stored at 4-30°C, in a cool, dark, and dry place. It has a shelf life of 24 months. The packaging should be opened in an appropriate temperature and humidity environment, and the test card should be used as soon as possible after opening.
Sample Requirements:
Nasal discharge, saliva, and ocular conjunctival secretions can be collected using a disposable swab. Samples can be stored at 2-8°C for up to 24 hours or at -20°C or lower for longer periods. Ensure that the sample temperature is restored to room temperature before use.
Testing Method:
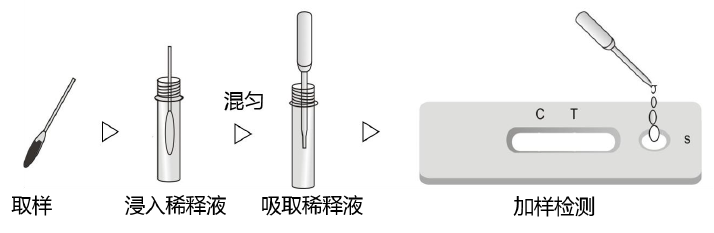
- Collect ocular conjunctival secretions, nasal discharge, or saliva using a cotton swab.
- Place the swab into a sample tube containing 1ml of sample diluent (do not mix sample diluents from different products).
- Rotate the sample tube containing the swab to thoroughly mix the sample and sample diluent.
- Slowly add 80ul (3-4 drops) of the mixed solution to the sample well.
- During the reaction, a red band will be visible moving in the result window of the test strip.
- Read the result after 15 minutes. Results read after 30 minutes are invalid.
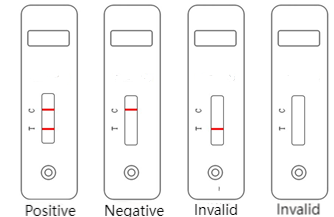
Interpretation of Test Results:
- Positive: A red band appears in both the test zone (T) and the control zone (C).
- Negative: Only a red band appears in the control zone (C), and no red band appears in the test zone (T).
- Invalid: No red band appears in the control zone (C), indicating an invalid test result. The sample should be retested.
Precautions:
- Follow the instructions carefully.
- Do not use tap water, purified water, or distilled water as a negative control.
- The color intensity of the band in the test zone (T) may vary. Within the specified observation time, even a very faint band should be considered a positive result.
- Sample collection, storage, and testing should strictly follow relevant biosafety guidelines.
- The test card is recommended for single use and should not be reused.
- The test result is for clinical reference only. Clinical diagnosis should consider symptoms, signs, medical history, and other laboratory tests.
- The desiccant inside the aluminum foil bag is not for consumption.
- Before testing, ensure that the sample and test card have reached room temperature. The recommended testing temperature is 18-30°C.

